Definitions of corrosion include:
− NACE/ASTM G193: ‘Corrosion is the deterioration of a material, usually a metal, that results from a chemical or electrochemical reaction with its environment.’
− ISO 8044: ‘Physicochemical interaction (often of an electrochemical nature) between a metal and its environment that results in changes in the properties of the metal, and which may lead to significant impairment of the function of the metal, the environment or the technical system, of which these form part.’
− NASA: ‘Corrosion can be defined as the degradation of a material due to a reaction with its environment. Degradation implies deterioration of physical properties of the material. This can be a weakening of the material due to loss of cross-sectional area, it can be the shattering of a metal due to hydrogen embrittlement, or it can be cracking of a polymer due to sunlight exposure. Materials can be metals, polymers (plastic, rubber, etc.), ceramics (concrete, brick, etc.) or composites (mechanical mixtures of two or more materials with different properties).’
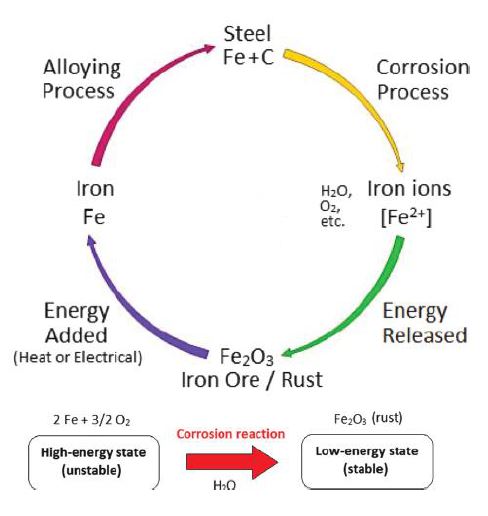
There are many forms of corrosion, but most follow the same cycle of returning the metal to its lowest energy state, as shown in below Figure.