Below table lists the crystal structures of the pure metals at room temperature. In nearly every case the metal atoms pack into the simple crystal structures of face-centered cubic (FCC), body-centered cubic (BCC) or close-packed hexagonal (CPH or HCP)
Some metals have more than one crystal structure. The most important examples are iron and titanium. Iron changes from BCC to FCC at 914 C but goes back to BCC at 1391 C. Titanium changes from HCP to BCC at 882 C. This multiplicity of crystal structures is called Allotropy. The phenomenon if not reversible, is termed Polymorphism and can be brought about at room temperature by alloying.
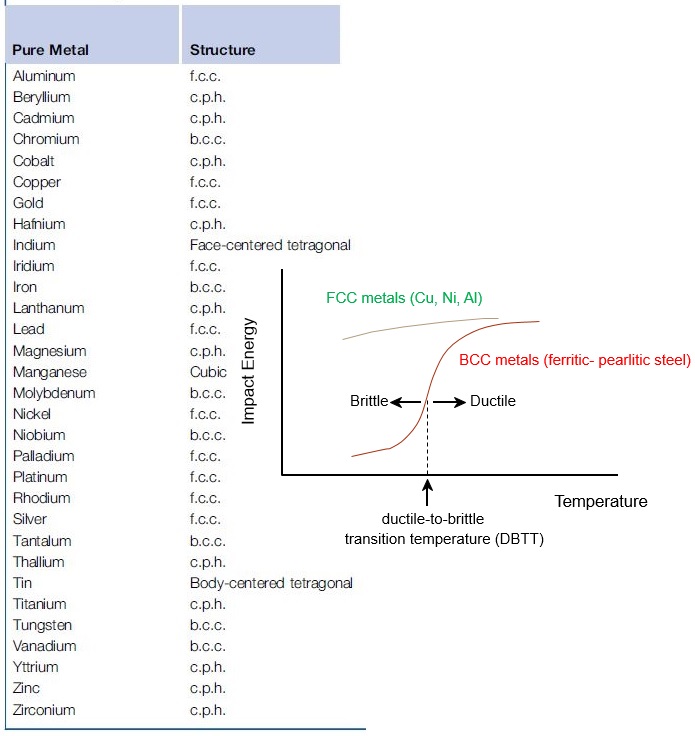
Indeed, the FCC metals such as austenitic stainless steel and aluminum alloys especially at low temperatures, have much better ductility and toughness than plain carbon steels and so good for cryogenic condition for storing liquid hydrogen, nitrogen, or oxygen.
But why?
In FCC metals, the flow stress, i.e. the force required to move dislocations, is not strongly temperature dependent. Therefore, dislocation movement remains high even at low temperatures and the material remains relatively ductile.
The yield stress or critical resolved shear stress of BCC crystals is markedly temperature dependent, in particular at low temperatures.
The temperature sensitivity of the yield stress of BCC crystals has been attributed to the presence of interstitial impurities on the one hand, and to a temperature dependent Peierls-Nabarro force on the other.