At 13.2 °C and below, pure tin transforms from the silvery, ductile metallic allotrope white tin (or β) having a body-centered tetragonal crystal structure to the brittle, nonmetallic, α-form grey tin with a diamond cubic structure.
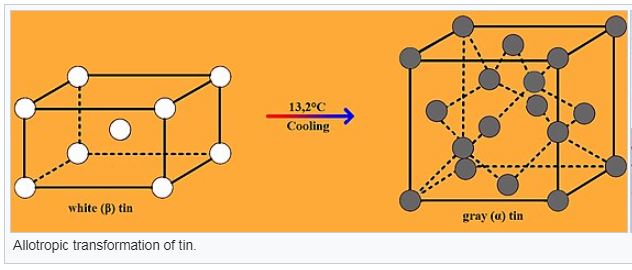
Accompanying this white to- gray-tin transformation is an increase in volume (27%), and, accordingly, this volume expansion results in the disintegration of the white tin metal into a coarse powder of the gray allotrope.
For normal subambient temperatures, there is no need to worry about this disintegration process for tin products, because of the very slow rate at which the transformation occurs.

This white-to-gray-tin transition produced some rather dramatic results in 1850 in Russia. The winter that year was particularly cold, and record low temperatures persisted for extended periods of time. The uniforms of some Russian soldiers had tin buttons, many of which crumbled because of these extreme cold conditions, as did also many of the tin church organ pipes. This problem came to be known as the “tin disease” or “tin pest.”
Another historical example: In 1910 British polar explorer Robert Scott hoped to be the first to reach the South Pole, but was beaten by Norwegian explorer Roald Amundsen. On foot, the expedition trudged through the frozen deserts of the Antarctic, marching for caches of food and kerosene deposited on the way. In early 1912, at the first cache, there was no kerosene; the cans – soldered with tin – were empty. The cause of the empty tins could have been related to tin pest.
Tin pest can be avoided by alloying with small amounts of electropositive metals or semimetals soluble in tin’s solid phase, e.g. antimony or bismuth, which prevents the phase change.